Workshop 2019
AgendaMarch 7, 2019

08:15 Coffee is served
08:30 Welcome – Opening Statements Torsten Schönberger (BKA) & Michael Maiwald (BAM)
08:50 Presentation: “Quality by Design (QbD) principles applied to NMR methods” Ian Clegg (Bruker Biospin Inc.)
09:10 Presentation: “Validation of Software” Carlos Amezcua (Baxter International Inc.)
09:30 Presentation: “Good Weighing Practice (GWP) for accurate qNMR sample preparation” Tucker Rubino (Mettler-Toledo GmbH)
09:50 Break
10:10 Workshop Session #1: Design of Analytical Procedures
(A) Establish an Analytical Target Profile (ATP) Session Leader: José G. Napolitano (AbbVie)
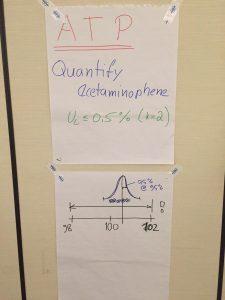
(B) Identify the Critical Quality Attributes of qNMR Session Leader: Dan Sorensen (Eurofins Alphora)

(C) Plan the Analytical Instrument Qualification Session Leader: Christoph Freudenberger (Bruker Biospin Inc.)


11:40 Presentation: “ValidNMR Website and Wiki” Kristie Adams (Steelyard Analytics)
12:00 Lunch
13:00 Presentation: “Tackling ‘dark’ uncertainty in SI traceable qNMR analysis of peptides and glycans” Cailean Clarkson (LGC)
13:20 Workshop Session #2: Verification of Analytical Procedures
(D) Plan the Analytical Control Strategy and Verification Session Leader: Torsten Schönberger (BKA)


(E) Experimental Factors to Consider for Generation of a qNMR Method Validation Protocol Session Leader: Joe Ray (Baxter International Inc.)

(F) Use of Statistical Tools for Evaluation of Measurement Uncertainty Session Leader: Michael Maiwald (BAM)

14:50 Open Forum – Q&A, Discussions ValidNMR committee members


15:15 The Workshop Adjourns – Closing Statements Torsten Schönberger (BKA) & Michael Maiwald (BAM)
15:30 ValidNMR Business Meeting ValidNMR committee members (open to observers and interested parties)
17:30 The ValidNMR Business Meeting Adjourns


